Energy and Thermodynamics
Heat Engines
By Mark Ciotola
First published on May 16, 2019. Last updated on July 30, 2024.
Introduction
A system that has pure physical aspects as well as social aspects is that of the engine. The Second Law of Thermodynamics tells that even the best real life heat engines will produce entropy along with work. No engine can produce work alone. (Thermal conduction itself results in lots of entropy production but little work).
The heat engine is a common example used to illustrate the Second Law of Thermodynamics. A heat engine utilizes a temperature difference (a thermodynamic potential) to perform work, such as moving a train or pumping water.
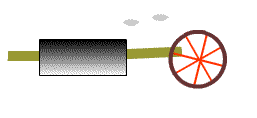
A heat engine
A simple heat engine is shown here. Most technical details have been omitted. Here, a tank of water that is presumably heated into high pressure steam by a flame or other energy source. The tank contains a piston that converts some of that heat energy into a cyclic in-out motion that represents work done upon a load, here represented by the wheel. The little puffs of steam represents the waste heat.
How A Heat Engine Functions
For a heat engine to function, heat must flow across a temperature difference from a warmer region to a cooler one. Such regions are called thermal reservoirs. Warmer heat reservoirs can be flames, hot air or steam, for example. Cooler heat reservoirs can be ice, cold air or cool water, for example. Air at room temperature can serve as either kind of reservoir depending on how hot or cold the other reservoir is.
The warmer region is typically called the hot reservoir (regardless of its actual temperature) with temperature \(T_H\), while the cooler region is designated as the cold reservoir with temperature \(T_C\). A typical heat engine undergoes a cycle of actions where heat flows into the engine, increases pressure of a fluid such as air or steam, used that pressure to push a piston, then releases the heat whereupon pressure is reduced. [1]
Example
The redder and higher the warmer heat reservoir, the hotter is it. Conversely, coolness is represented by bluer shading and lower height. The bluer and lower the cooler heat reservoir, the colder it is. Our heat engine begins operating between a quite hot and a quite cold reservoir as shown here.
Here we see a heat engine working between a warmer reservoir and a cooler reservoir (below). An example of this scenario is the temperature difference between a hot flame and a cool tank of water being used in a steam engine. When thermal energy flows to power a heat engine, part of the available energy is put into work and the remainder results in waste heat.
Below, the lefthand red area represents the hot reservoir, and the righthand blue area represents the cold reservoir. Warmth is represented by redder shading (if your version is in color) and greater height. The gray area represents the engine. Heat flows through the engine, which does work by turning a wheel, which might drive a generator, push a car or run a machine tool.
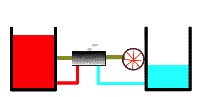
Heat engine operating upon a thermal potential
Efficiency
No engine turns all of the heat flow into work. That would imply 100% efficiency, which is impossible in theory as well as practice, regardless of how well the engine is constructed. The Second Law of Thermodynamics tells that even the best engines will produce entropy along with work. Efficiency \(\epsilon\) can be expressed as:
\(\epsilon = \frac{Q_H – W}{Q_H}\)
where \(Q_H\) is the flow of thermal energy out of the hot reservoir and \(W\) is work.
Carnot Efficiency
The best efficiency that an ideal engine can achieve is known as its Carnot Efficiency. The efficiency of a Carnot Engine depends upon the temperature difference between the thermal reservoirs, where the temperature of the hot reservoir is Thand that of the cold reservoir is Tc. The Carnot Efficiency \(\epsilon_c\) is simply the difference between the warmer and cooler temperature divided by the warmer temperature:
\(\epsilon_c = 1 – \frac{T_C}{T_H}\)
Calculating the Carnot Efficiency must be done using absolute temperatures, that is, temperatures measured from absolute zero. Absolute zero is the lowest possible temperature in theory, and has never been quite obtained in practice. Such temperatures are measured in a kind of degree called Kelvin. 0° Celsius equals about 273.15 Kelvin.
An example is the temperature difference between a hot flame and a cool tank of water being used in a steam engine. Then, part of the available energy is used to perform work and the remainder is exhausted as waste heat. For instance, a steam engine could contain a piston that converts some of the heat flow into a cyclic in-out motion that represents work done upon a load, such as a flywheel wheel. Steam released into cooler air represents waste heat. When waste heat is created, an intangible quantity called entropy is produced. The more the heat engine works, the more entropy it will produce.[2]
Work Performed by a Carnot Engine
If an amount of heat \(Q_H\) is removed from the hot reservoir, the the amount of work \(W\) a Carnot engine will perform is
\(W = \epsilon~Q_H\).
Work Performed by Real Life Heat Engines
In reality, most engines are a great deal less efficient than even the Carnot efficiency. While a Carnot Engine can be approximated in real life, despite its high efficiency, it functions too slowly to be of much value for real life uses. Practical heat engines tend to operate of much lower efficiencies. This real life efficiency is called “Second Law Efficiency”, \(\epsilon_s\).
\(\epsilon_{Second~Law} \geq \epsilon_{Carnot}\).
One reason that engines operate at lower efficiencies than better construction would allow is that more efficient heat engines tend to take more resources to build.
Further Discussion
If heat flows from a warmer object to a cooler object (where no engine is involved), no work results, but entropy is still produced (or you could say that the entropy of the system under consideration increases). Thermal conduction itself results in lots of entropy production but little work. A thermal conductor can be thought of as a lazy heat engine. Chemical reactions, such as burning coal and oil or metabolizing sugars also results in entropy production. The Second Law of Thermodynamics states that overall entropy (of an entire system) will tend to increase.
Notes & References
[1] For the origins of heat engine theory, read the works of Sadi Carnot.
[2]In theory, a heat engine is not required to produce entropy if the temperature of the cold region is absolute zero (which is about – 273° C). In practice, such a low temperature is physically impossible.
« Second Law of Thermodynamics | COURSE | Heat Engine with Exhaustible Reservoirs »